1. Introduction
Thermal plasma enables rapid evaporation rate, abrupt temperature gradients, and high chemical reaction, and is therefore a fascinating route for the fabrication of nanomaterials. Using direct current (DC) arc plasma for nanomaterial production has several advantages. First, this method is an environmentally friendly technique because DC arc plasmas do not generate toxic by-products or hazardous gases. Second, DC arc plasma can be used for melting refractory metals because of its high temperature. Finally, a high purity nanoparticle can be continuously produced. The DC arc plasma has been used for the synthesis of various nanomaterials, such as carbon [
1,
2,
3], alloy [
4,
5], oxide [
6,
7], intermetallic compound [
8], and high-purity metal nanopowders [
9,
10].
In the arc discharge process, the raw material is offered by the anode when preparing the nanoparticles, and the productivity of the nanoparticle depends on the anode consumption rate. Accordingly, the anodic region and anode phenomena need to be further analyzed to increase the yield and control nanoparticle size. In fact, the anode boundary has three typical arc-anode adhesion modes, namely, diffusion, multiple, and constricted attachments [
11,
12]. Different arc-anode attachment modes contribute to the competition between the cathode and anode jets. Nevertheless, an in-depth research of the arc-anode attachment modes in argon-hydrogen arcs for nanoparticle production has not been conducted, and thus the arc discharge method is rarely used in nanoparticle production.
Considerable attention has been drawn to nickel nanoparticles due to their excellent chemical, physical, and electronic properties [
13,
14]. Recent research tendencies have focused on energy conversion and storage devices including metal-air batteries, fuel cells and supercapacitors, in which the supercapacitors are characterized by their high performance in terms of energy and power density, simple operating conditions and long cycling life [
15,
16,
17]. Besides, Na-air batteries have gained much attention as next generation power storage because of their high theoretical specific density and capacity. Nickel nanoparticles are potential catalysts due to their large surface areas and additional active sites. Nickel nanoparticles catalyze the transfer hydrogenation of olefins and carbonyl compounds and the reductive amination of aldehydes with 2-propanol as hydrogen donor [
18]. However, the use of nickel nanoparticles as catalysts for the oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) for metal-air batteries is rarely reported. Recently, hybrid sodium-air batteries have potential application in electric vehicles due to their high energy density and low cost [
19,
20]. Therefore, developing a cheap non-noble metal catalyst for hybrid sodium-air battery is necessary.
In the present work, nickel nanoparticles were prepared through DC arc discharge, and diffusion, multiple, and constricted attachment modes were observed in argon-hydrogen arcs. The effect of arc-anode attachment mode on the diameter of nanoparticles and their size distribution is investigated by using a high-speed camera and current/voltage measurement. Nickel nanoparticle generated by DC arc discharge in 50% H2 concentration has high productivity, fine crystallinity, and appropriate size distribution. Therefore, the nickel nanoparticles prepared under 50 vol% H2 were used in hybrid sodium-air batteries and displayed good catalytic performance, which is comparable to commercial silver nanoparticles.
3. Results and Discussion
Figure 2 shows the snapshots of a high-speed camera of argon arc with different H
2 concentrations of 0, 30, and 50 vol%, corresponding to
Figure 2a–c, respectively. We assumed that the cathode and anode jets are composed of hydrogen and nickel, respectively. Three typical arc-anode attachment modes were observed in argon arc at different H
2 concentrations.
Figure 2a illustrates that the cathode jet impingements on the anode surface form a stagnation layer in front of the anode, which contributes to the well-known bell shape of the arc. In this case, the anode arc root is rather diffuse. This arc-anode attachment mode is expressed as a diffusion attachment mode [
12]. Several small anode spots at the anode boundary can be seen at the same time in
Figure 2b, which is the feature of multiple attachment modes [
13].
Figure 2c presents the constricted attachment mode. The original reason of different arc-anode attachment modes is the evaporation of anode. The competition of the cathode jet and the anode jet leads to different forms of arc-anode attachment mode [
10]. Multiple attachment modes have been considered to be transition modes between diffuse and contractile modes. The transition of the attachment mode is mainly related to the variation of anode evaporation rate caused by different hydrogen concentrations.
No anode jet was observed in pure Ar conditions, and thus we used a high-speed camera with the band-pass filter to observe the cathode and anode jets when the hydrogen concentrations were 30 and 50 vol%.
Figure 3a shows the snapshots of the high-speed camera of argon arc when hydrogen concentration is 30 vol%. The cathode jet is on the top of anode jet in this figure. From these snapshots, the multiple attachments can be concluded to form at the anode jet region with 30 vol% of hydrogen. These images are used for the illustration of the variation in the anode and cathode jet areas.
Figure 3b exhibits the current and voltage waveforms synchronized with snapshots. In multiple attachment modes, the area of the hydrogen cathode jet at the peak value of the arc current is larger than that of the arc valley. On the contrary, the hydrogen anode jet is larger at the valley of the arc current than that at the peak of the arc current. The reason is that the high arc current is helpful in enhancing the cathode jet, but the anode jet decreases due to the balance between the cathode and anode jets.
Figure 4a shows the snapshots of the high-speed camera for argon arc with 50 vol% of hydrogen. Constricted mode can be clearly seen at the anode boundary. The area of the nickel anode jet increases with increasing hydrogen concentration, whereas the hydrogen cathode jet area drops with increasing hydrogen concentration due to the strong intensity of the cathode jet. This result can be explained by the competition between cathode jet and anode jet [
10].
Figure 4b presents the current and voltage waveforms of argon arc with 50 vol% of hydrogen synchronized with the arc behavior snapshots. Noticeably, the relatively large nickel anode jet is observed at the peak of the arc current. In addition, significant movement of anode spot can be seen in the constricted mode, which is also confirmed by the large fluctuation of voltage waveform. However, no clear relationship is observed between the arc current and the area of the anode jet due to the instability of the arc.
Figure 5 shows the relationship between the anode and cathode jet areas (upper) of argon arc with different hydrogen concentrations and the time variation of the current and voltage waveforms of synchronous arc (lower).
Figure 5a-a1 and b-b1 illustrate the hydrogen concentrations of 30 and 50 vol%, respectively.
In the waveform, the peak of cathode jet area variation and the valley of the anode jet area variation appear nearly simultaneously. The average areas of the cathode jet are 2850 and 4000 pixels, corresponding to the hydrogen concentrations of 30 and 50 vol%, respectively.
Figure 5 show that the waveform of the cathode jet variation follows the shape of the arc current in multiple attachment modes at 30 vol% of hydrogen. By contrast, no clear relationship between the arc current and the area of the anode jet in the constricted mode is found. The variations in the cathode and anode jet areas show large fluctuations in the constricted mode, and these fluctuations are attributed to the instability of the arc. In addition, the noise in voltage waveform can be explained by the instability of the arc. Hydrogen concentration plays a critical role in the formation of different attachment modes, and a high hydrogen concentration contributes to the formation of constricted mode due to the large evaporation rate.
Figure 6 presents the XRD patterns of the synthesized nickel nanoparticles in the argon arc with different hydrogen concentrations. Three typical diffraction peaks of the patterns can be fully indexed into (111), (200), and (220) planes of nickel. The diffraction peaks of the nickel nanoparticles generated in 100 vol% Ar can be found to be weak and broad, which indicates a weak crystallinity of the nickel nanoparticles with a relatively small diameter. With the increasing concentration of hydrogen, the diffraction of peaks becomes sharper and stronger, indicating an improved crystallinity and large particle size. When the hydrogen concentration is 50 vol%, the diffraction of peaks is the sharpest and strongest, contributing to the best crystallinity of nickel nanoparticles with relatively large particle size. According to Scherrer equation, the average grain size of based on the (111) diffraction peaks for the samples with Ar, 30 vol% H
2, 50 vol% H
2 are 20 nm, 34 nm, 59 nm, respectively. Compared with other outputs of 0.01 g/min, 0.03 g/min at the hydrogen concentration of 0 vol% and 30 vol%, the highest output of 0.3 g/min is obtained when the hydrogen concentration is 50 vol%, because a high hydrogen concentration leads to high evaporation.
Figure 7 indicates the TEM images of nickel nanoparticles obtained at different hydrogen concentrations from 0 vol% to 50 vol%.
Figure 7a shows that nanoparticles are dominated by a spherical shape besides some irregular shapes when the hydrogen concentration is 0 vol%.
Figure 7b,c illustrate that all the nanoparticles are spherical. In comparison with nanoparticles obtained in diffuse and multiple modes, the particle size becomes larger in constricted mode.
Figure 7a1–c1 reveal the corresponding particle size distributions. The mean diameters of nanoparticles are 20, 33, and 63 nm, corresponding to the hydrogen concentrations of 0, 30, and 50 vol%, respectively. As the evaporation rate of nickel anode increases, the average particle size increases with the increase of hydrogen concentration. The particle diameter is strongly affected by the number species of nickel vapor. A high nickel vapor concentration would enhance the nanoparticle growth by condensation, resulting in large particle size distribution. In the case of higher hydrogen concentration, the evaporation of nickel metal was enhanced by thermal pinch effect. According to the formation mechanism of nanoparticles by arc discharge (
Figure S2), the metal vapor will grow into nanoparticles by condensation and collision after nucleation. Higher concentration of metal vapor leads to larger particle size by condensation. Therefore, the higher hydrogen concentration contributes to larger average particle size. The nanoparticle size can be controlled by the following strategies, modification of the power supply by using capacitor or coil, using shield gas around the cathode [
10], selecting plasma gas which contributes to form constricted arc, using hollow cathode [
12] and rotating anode [
7].
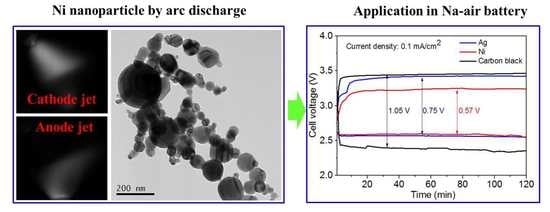
Figure 8 presents the discharge-charge curves of the hybrid Na-air battery with nickel nanoparticles, silver nanoparticles, and carbon black catalysts. The current density is 0.1 mA/cm
2 and the discharging-charging time is 2 h.
Figure 8 shows that the battery using carbon black as catalyst displayed a discharge voltage of 2.38 V, corresponding to 76.53% of the theoretical voltage. Carbon black has a relatively poor catalytic performance due to its limited specific surface area and few activation sites [
23]. The hybrid Na-air battery using silver nanoparticles has a discharge voltage of 2.63 V. Notably, a high charge voltage of 3.38 V is obtained due to the limitation of the catalytic performance of OER, resulting in a voltage gap of 0.75 V. Nevertheless, the hybrid Na-air battery using nickel nanoparticles as catalysts displays a higher discharge voltage of 2.65 V and lower charge voltage of 3.22 V than that using commercial silver nanoparticle, which results in a lower voltage gap of 0.57 V, corresponding to a round-trip efficiency of 80.3%. As shown in
Figure S3, nickel nanoparticles show better electrochemical performances at different discharge current densities than Ag nanoparticles and carbon black. The good catalytic performances of nickel nanoparticles prepared by DC arc in 50% hydrogen concentration are ascribed to good conductivity and additional activation sites induced by good crystallinity and appropriate particle size distribution. Moreover, nickel nanoparticles may be polycrystalline, and the surfaces seen from TEM images are covered with amorphous NiO layers (
Figure S4a). The NiO layer was also confirmed by XPS results in
Figure S5. NiO has typical peaks for Ni
2+ in NiO: 855.3 eV for Ni
2+ 2p3/2 [
24,
25]. The composite of Ni/NiO contributes to their good catalytic performances for OER and ORR by the low charge transfer resistance of NiO [
26].
Figure 9 illustrates the cycling performance of the hybrid Na-air battery with nickel nanoparticles as catalysts. The battery cycles for 20 min at the current density of 0.1 mA/cm
2 of discharging and charging per cycle. The high charge-discharge efficiency and good durability for Ni nanoparticles as catalysts during 100 cycles are attributed to two reasons. First, Ni nanoparticles with good conductivity, additional activation sites, and Ni/NiO composite structure contribute to their good catalytic performances. Second, the appropriate particle size distribution leads to the good stability of nickel nanoparticles as catalysts in the hybrid Na-air battery. During the first 50 charge-discharge processes, the tripping efficiency decreases from 80.3% to 80.0%. This result indicates that the hybrid Na-air battery using nickel nanoparticles as catalysts has a good cycling performance [
21]. Nevertheless, from the 50th to the 100th charge-discharge process, the round-trip efficiency drops from 80.0% to 69.3% due to the evaporation of NaOH. The limited volume of NaOH solution is one restricted factor in this experiment (around 0.2 mL of NaOH) and leads to an increase in the NaOH concentration, even with the separation of the electrolyte and catalyst between the aqueous solutions [
19]. The high solubility of oxygen under alkaline condition and the increase of the NaOH concentration enhance the internal resistance of the battery [
20].
4. Conclusions
Nickel nanoparticles were prepared by the DC arc discharge method. Argon and argon/hydrogen mixtures were used as plasma gas. The hydrogen concentrations of 0, 30, and 50 vol% in the argon arc correspond to diffuse, multiple, and constricted arc-anode attachments, respectively. We investigated the effects of hydrogen concentration on the formation of different arc-anode attachment modes, understanding the arc phenomena contributes to the effective preparation of nanoparticles. Nickel nanoparticle generated by DC arc discharge in 50 vol% H2 concentration has high productivity, fine crystallinity, and appropriate size distribution. The large evaporation of the anode material leads to the formation of constricted anode attachment in the case of high hydrogen concentration. The variation waveform of the cathode jet follows the arc current shape in multiple arc-anode attachment modes at 30 vol% of hydrogen. Meanwhile, no clear relationship is found between the arc current and the area of the anode jet in the constricted mode. In comparison with nanoparticles obtained in diffuse and multiple modes, the particle size becomes larger in the constricted mode due to the large metal vapor concentration. The mean diameters of nanoparticles are 20, 33, and 63 nm, corresponding to the diffuse, multiple, and constricted arc-anode attachments, respectively.
Nickel nanoparticles generated by 50 vol% of hydrogen concentration were used as catalysts for a hybrid sodium–air battery. Nickel nanoparticles, owing to their good crystallinity, additional activation sites, and Ni/NiO composite structured, have relatively better catalytic performance than commercial silver nanoparticles and carbon black. Nickel nanoparticles used as catalysts display 0.57 V voltage gap at the current density of 0.1 mA/cm2, which is lower than silver nanoparticles and carbon black corresponding to 0.75 V and 1.05 V, respectively. Nickel nanoparticles demonstrate good stability like the catalysts in hybrid sodium-air battery. As nickel nanoparticles possess a lower cost than silver nanoparticles and other noble metals, nanoparticles generated by arc discharge method are promising catalysts for hybrid sodium-air batteries and other alkaline metal-air batteries used in large-scale applications.